Molecules that can adopt multiple conformations may show multiple peaks in NMR spectra. One way to test if the extra peaks in your spectrum are due to conformational isomers is to change the temperature. Changing the temperature will change the rate of exchange between the conformations and alter the chemical shifts. In addition to confirming the cause of the extra peaks variable temperature NMR may also provide thermodynamic information. An example based on the thyroid hormone thyroxine is described below.
Thyroxine is an iodinated derivative of tyrosine found in all vertebrates. The bulky iodine atoms on thyroxine force its aromatic rings into a perpendicular orientation that places one proton on the hydroxyl bearing ring over the center of the other ring, the "proximal" position (H2'), while the other proton is placed in the "distal" position (H6').
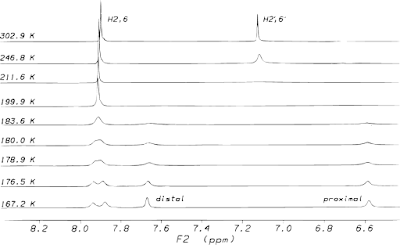
In this orientation one would expect that the H2' and H6' protons would give two different peaks due to ring current effects, but this is not the case. At room temperature the 1H NMR spectrum shows just one signal from the two protons, near 7.10 ppm. If the temperature is lowered, however, these signals separate. This indicates that at room temperature the environments of the protons on the aromatic rings are exchanging rapidly enough that only an averaged resonance can be detected.
If you have multiple resonances in your spectrum at room temperature, and increasing the temperature causes those peaks to move towards each other and coalesce, then this is good evidence that the peaks are due to conformational isomerism. To eliminate the possibility that you have temperature dependent chemical shifts you should increase the temperature beyond the coalescence point and see only a single peak that does not move. The graph below of the thyroxine data shows that the coalesced H2',6' peak does not shift as the temperature is increased.
This collection of spectra at different temperatures can also provide additional information. If you can determine the maximum separation of the peaks (δυ), and the temperature at which they coalesce (Tc), then you can determine the energy barrier (ΔG≠) for the exchange process using the following equation.
ΔG≠ = 19.14 Tc[9.97 + log(Tc/δυ)]
In the case of thyroxine, the energy barrier for exchange of the proximal and distal environments of the H2',6' protons was found to be around 37 kJ/mol. Interestingly, the energy barrier was the same for the separation of the H2,6 protons on the other aromatic ring, suggesting that it is the same process affecting both rings. This thermodynamic information may be useful for designing molecules that adopt a specific conformation.
References
B.M. Duggan and D.J. Craik
"Conformational dynamics of thyroid hormones by variable temperature nuclear magnetic resonance: the role of side chain rotations and cisoid/transoid interconversions"
J Med Chem 1997 40(14):2259-65.



No comments:
Post a Comment