Late last year Prof Mike Gilson sent me a Science paper that reported "boosted mobility" of molecules during reactions. The conclusions were based in part on DOSY experiments and since the reported increases were greater than expected, Prof Gilson was interested in a second opinion on the NMR experiments. As I started looking at the data a technical comment disputing the results, and a response from the original authors was published. The original group then published another paper addressing the problems raised by the second group and made their raw NMR data freely available. A few weeks ago the second group published a preprint where they argue the first group's data was not analysed correctly and the conclusions are invalid. In this post I'll go through the data from both groups, my own analysis of the data, and try to make some sense of it all.
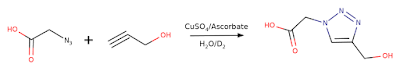
The original paper from the lab of Steve Granick1 set up a variety of reactions under conditions where the reactions take several hours to complete. By performing the reactions in an NMR tube a series of DOSY experiments was recorded so that the diffusion coefficients of the reactants, products and solvent could all be monitored as the reaction proceeded. Some reactions, including the click chemistry reaction shown below, were reported to show increased diffusion at the start of the experiment that decreased as the reaction proceeded.
The technical comment from the lab of Jonathon Beeves2 disputed these results, stating that the DOSY results were inaccurate because the DOSY gradient strengths were increased linearly, allowing other processes occurring during the reaction to confound the results. It must be said that linearly increasing DOSY gradient strengths is standard practice, but in the similar protocol of recording NMR relaxation data the order is randomised href reduce the impact of any systematic variation during the course of the experiment.
Granick's lab disputed Beves' interpretation3 and published a second paper4 that sought to determine if the order of the gradients affected the results by repeating the experiments with linearly increasing gradients, linearly decreasing gradients, and a randomised order of gradients. Figure 2E from this paper, reproduced below, shows their results. Panels b, c and d show the change in the diffusion coefficients measured over the course of the click reaction for the reactant alkyne methylene signal, while panels f, g and h show the change for the reactant azide methylene. Panels b and f show data obtained using decreasing gradient strengths, panels c and g for random ordered gradients, and d and h for increasing gradients (as used in the first paper).
The increasing gradients show larger initial diffusion coefficients than the decreasing or randomised gradients, suggesting increasing gradients overestimate the diffusion coefficients. Panels a and e plot the ratio of diffusion coefficients from decreasing or randomised gradients to those from increasing gradients. To my eye panels a and e indicate that the decreasing and random gradients give lower values at the start of the experiment and higher values at the end, relative to the increasing gradients, but the paper states the ratio "is unity within experimental uncertainty at all reaction times".
The raw NMR DOSY data for panels c and g was made publicly available5 so I attempted to process and reproduce the graphs in the paper. In my analysis the errors were smaller than the points used to plot the values, so I did not include error bars. As an aside, its not clear to me why random gradients should lead to larger uncertainties, as reported, unless the order is influencing the results. My analysis of the publicly available data, shown below, shows the same trends as those published by the Granick lab, but the scales are different.
Their reaction time scale only goes to 150 minutes but the publicly available data covers 247 minutes in 61 DOSY experiments. It is not possible to follow all peaks all the way through the reaction because they move and broaden and may overlap other peaks. This is why the reaction time scale on my azide methylene data (right panel above) does not go to 250 minutes, but I was able to follow the alkyne methylene peak through the full 247 minutes. The vertical scale is more problematic, as this is the point of the original paper. In my analysis the diffusion coefficient was only increased by 25%, not 50% as reported.
The Granick lab also made the data for Figure 3 from their second paper available. In the figure below the top two panels labelled A and B are from the paper and the lower two graphs are my attempts to reproduce them.
Once again the trends are similar but the scales do not seem to match. The publicly available data provided 30 DOSY experiments covering 108 minutes, but the data in the paper covers 250 minutes. It seems unlikely that the Figure 2 and 3 datasets were swapped because the trends for the azide diffusion data match.
The Beeves lab also analysed the publicly available data and published a preprint6 listing several points that may have affected the results. They point out that the Granick lab normalised their diffusion data to the final measured diffusion value, called "D0" by the Granick lab. Since the diffusion data cannot be followed all the way through the reaction for all peaks, the diffusion coefficients for different peaks are normalised to values obtained at different times in the reaction. The top panel of the figure below from the Beves lab preprint shows how the final diffusion value, called "D∞" by Beves, differs in different experiments.
To eliminate this variation the Beeves lab measured diffusion coefficients of the click reaction mixture without the catalyst, which they called "D0". They found these diffusion coefficients to be greater than those measured during the reaction. The lower panel of the figure above shows that normalising to values obtained before the reaction started (i.e. "Dnorm=D0") showed an apparent decrease in diffusion over the course of the reaction. Normalising to the final diffusion value (i.e. "Dnorm=D∞", as was done by the Granick lab) shows boosted mobility at the start of the reaction.
Prof Gilson found literature diffusion coefficients for 2-propanol in water7, a molecule similar to the alkyne used in the click reaction. At 14x10-10 m2/s this was larger than the alkyne diffusion coefficient measured by Beves (10.4x10-10 m2/s) and that measured by Granick (5.5x10-10 m2/s). While it is not the same molecule, it does suggest that the normalisation values used by the Granick lab are low.
At this point it seems to me that while there may be some increase in diffusion during reactions its unlikely to be as large as reported by the Granick lab. I am sure there will be more publications on this topic appearing soon.
References
1. Boosted molecular mobility during common chemical reactions.
Wang H, Park M, Dong R, Kim J, Cho YK, Tlusty T, Granick S.
Science. 2020 Jul 31;369(6503):537-541
2. Comment on “Boosted molecular mobility during common chemical reactions”
Günther JP, Fillbrook LL, MacDonald TSC, Majer G, Price WS, Fischer P, Beves JE.
Science. 2021 Jan 15;371(6526):eabe8322
3. Response to Comment on “Boosted molecular mobility during common chemical reactions”
Wang H, Park M, Dong R, Kim J, Cho YK, Tlusty T, Granick S.
Science. 2021 Jan 15;371(6526):eabe8678
4. Using NMR to Test Molecular Mobility during a Chemical Reaction
Huan Wang, Tian Huang, and Steve Granick
J Phys Chem Lett. 2021 Mar 11;12(9):2370-2375
5. Use or misuse of NMR to test molecular mobility during chemical reactions
Huan Wang, Tian Huang, and Steve Granick
Zenodo, January 27, 2021
6. Errors in the Use of NMR to Test Molecular Mobility during a Chemical Reaction
Lucy Fillbrook, Jan-Philipp Günther, Günter Majer, William S. Price, Peer Fischer, Jonathon Beves
chemRxiv Pub Date : 2021-03-29
7. The mutual diffusion coefficient for binary mixtures of water and the isomers of propanol
K. C. Pratt and W. A. Wakeham
Proc. R. Soc. Lond. A 1975 Mar 25;342:401–419





As I was organising the references for this post I discovered that the publicly available Granick data had been updated. I had used the first version for my analysis so it seems likely it does not correspond to the data in the J Phys Chem Lett paper making my analysis irrelevant. The Beves lab analysis seems to have used the updated data.
ReplyDeleteI want to thank Brendan for taking an interest in this unusual problem and interpretation challenge, and for his insightful analysis of the data.
ReplyDeleteIn case you are interested in more background in the phenomenon in question, also known as enhanced enzyme diffusion (EED), Mudong Feng in my lab and I wrote a review on this subject about a year ago. doi:10.1146/annurev-biophys-121219-081535